We use cookies on your browser to customize content for your viewing and for analytics. If you click “Accept” or continue to browse our website, we assume you have agreed to our use of cookies. For details, please refer to our Cookies Policy.
02 December
151
A paper from JACC, it demonstrated that Pulmonary Artery Denervation (PADN) induces permanent Sympathetic Nerves(SN) injury and improves Hemodynamic and Pulmonary Artery (PA) remodeling, and explores specific sites of SN distribution and ablation facilitated by PADN.

In 28 December, the Division of Cardiology, Nanjing First Hospital, Nanjing Medical University, Department of Cardiovascular Research, Nanjing Medical University, Division of Cardiology, Nanjing Heart Center, and the Department of Cardiovascular Science, University of Sheffield, Sheffield, United Kingdom,published a research that the critical role of Pulmonary Artery Denervation (PADN) in mitigating pulmonary arterial hypertension (PAH) by targeting sympathetic nerve (SN) overactivity.
Study Background
Pulmonary arterial hypertension (PAH) is a diverse group of diseases characterized by progressive pulmonary vascular remodeling and right ventricular(RV) failure. Current treatments are limited to pharmacological vasodilation and antiproliferation; however, persistent pulmonary artery (PA) remodeling puts many patients at risk of death.The innervation of the PA is predominantly sympathetic, and increased sympathetic nerve (SN) activity and circulating catecholamines have been demonstrated in patients with PAH, suggesting that SN overactivation plays a critical role in PAH. Previous studies have reported short-term improvements in pulmonary arterial pressure (PAP) and cardiac function by PADN.
Study Method
In this study, 40 adult beagle dogs were randomly assigned into control and test groups.
The test group received an intra-atrial injection of dehydrogenized monocrotaline (DHMCT) to induce PAH. After eight weeks, dogs with mean pulmonary arterial pressure (mPAP) >25 mm Hg were divided into sham and PADN groups. At 14 weeks, the hemodynamics, medial wall thickness and PA muscularization, and messenger ribonucleic acid expression of genes in lung tissues were measured. Another 35 PAH dogs were used to measure the SN conduction velocity, electron microscopic assessment, and nerve distribution.
Results
PAH MODEL AND MORTALITY.
Eight weeks after injection with DHMCT, the PAH model was successfully established in 20dogs (67%) in the test group. A total of 4 deaths occurred after the second randomization, with 3 in the sham group and 1 in the PADN group. Finally, the sham group contained 7 dogs, and the PADN group contained 9 animals.
PADN PROCEDURE.
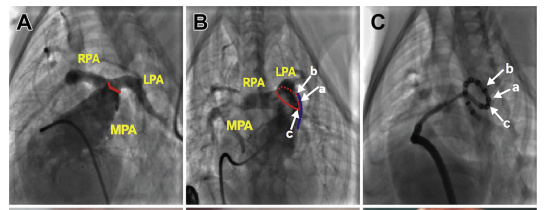
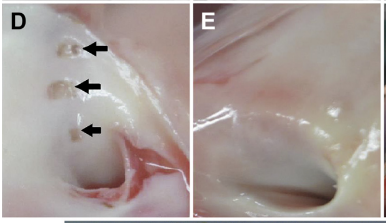
PADN was performed at 3 sites(Figure A\B\C). The median procedural time was 7.5 min (interquartile range: 6 to 16 min). Immediately after PADN, the ablated sites were clearly observed on the intima of the PA (Figure D); these sites were sealed and replaced by a scar at 1 month (Figure E). No procedure-related complications occurred.
VISUAL ASSESSMENT OF SN INJURY.
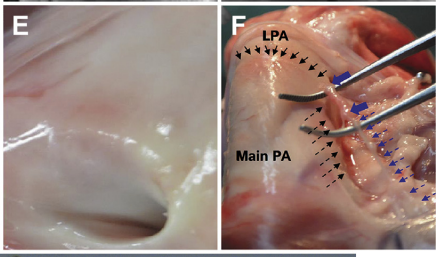
The noninjured SN had a smooth surface (Figure F, dashed blue arrows). Immediately after PADN, the ablated segment became swollen (Figure F, solid blue arrows), which was in line with an intimal injury (Figure E)
DISTRIBUTION OF SYMPATHETIC NERVES.
PA sympathetic nerve bundles were localized in the groove parallel to the left lateral side of the main PA trunk (Figure F) and were mostly bifurcated into the posterior wall of the main PA and LPA, with very few in the anterior wall of the right PA. The shortest distance from the nerves to the PA lumen was 823 mm, and the longest distance was 4,148 mm (Figures G to I).
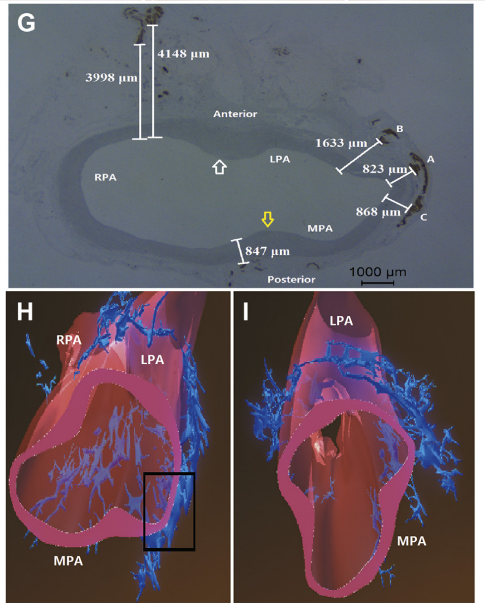
SNCV MEASUREMENTS.
After the PADN procedure, the SNCV decreased very rapidly over time.
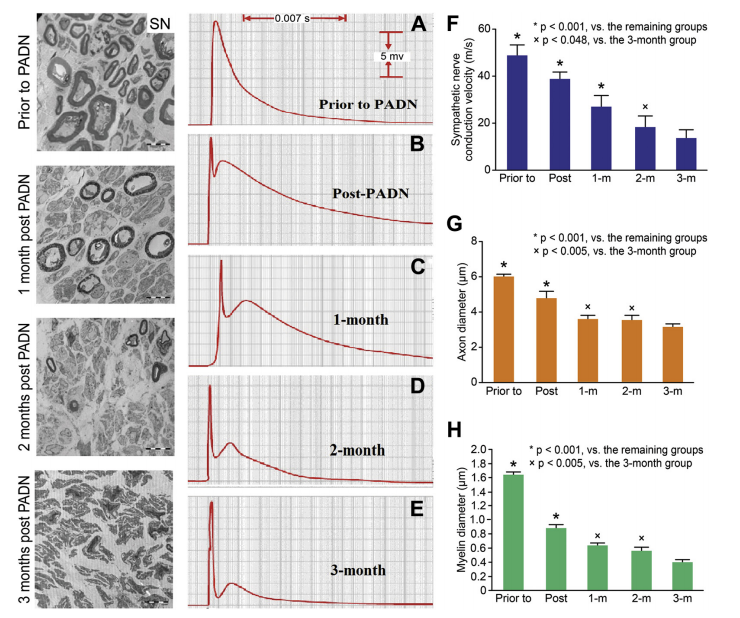
ELECTRON MICROSCOPIC ASSESSMENT OF SN.
Most nerve endings in animals with mPAP >25mm Hg had smooth and relatively thick myelin, significantly higher than those in animals with mPAP ≤25 mm Hg . After the PADN procedure, the myelin sheath became noticeably thinner and the vacuoles degenerated over time ,with axon diameters significantly decreased to 3.53 atrial pressure, and increased CO(cardiac output), leading to a significant reduction of pulmonary vessel resistance compared with the sham group. Finally, RV hypertrophy, by means of the ratio of the RV mass/(left ventricle þ septal), in the PADN group was significantly lower than in the sham group.
PA REMODELING.
The %MWT in the PADN group was 22.3±3.3%, significantly lower than that in the sham group (30.4±4.1%, p < 0.001).The rates of NM and full muscularization in the Shamgroup were 12.9±4.9% and 57.1±5.7%, respectively,significantly different from the PADN group 29.8 6.1% and 40.3±9.3%, respectively (all p < 0.001). There was no significant difference in partial muscularization between the sham and PADN (p ¼ 0.057) groups.
REAL-TIME QUANTITATIVE POLYMERASE CHAIN REACTION.
DHMCT-exposure was characterized by overexpression of platelet-derived growth factorsubunit B, fibroblast growth factor-2, endothelin-1,endothelial nitric oxide synthase, and monocyte chemoattractant protein-1. These alterations were significantly less in the treatment group.
Conclusions
This study confirms that PADN induces sustained sympathetic nerve injury, resulting in significant hemodynamic and pathological improvements in pulmonary arteries affected by PAH. By mapping the precise distribution of SNs and optimizing ablation points, PADN effectively remodels pulmonary arteries and reduces pulmonary arterial pressure. These findings pave the way for further experimental and clinical exploration of PADN as a viable treatment for PAH, emphasizing the importance of targeting SN activity and distribution in the management of this condition. The promising results highlight PADN's potential to revolutionize PAH therapy by addressing the underlying mechanisms of pulmonary artery remodeling and sympathetic nerve overactivity.
About PADN
PADN (Pulmonary Artery Denervation) is a groundbreaking and highly innovative radiofrequency ablation device for the treatment of pulmonary hypertension (PH). A potential first-in-class and best-in-class device, PADN uses vascular intervention to ablate the pulmonary sympathetic nerve, effectively reducing pulmonary artery pressure and slowing the progression of the disease.
The innovative nature of PADN is underscored by its designation as a Breakthrough Device by the US FDA in 2021, a recognition reserved for devices that have the potential to offer more effective treatment for life-threatening or irreversibly debilitating conditions. In 2023, the PADN received FDA Humanitarian Use Device (HUD) certification and National Medical Products Administration (NMPA) approval, highlighting its promise in the treatment of PH conditions.
About Pulnovo Medical
Pulnovo Medical Limited, is a globally recognized device pioneer in the treatment for pulmonary hypertension and heart failure. Established in 2013 and rooted in innovation, Pulnovo Medical is committed to leveraging our deep expertise in the science of breakthrough technologie with the goal to market our innovative therapeutic solutions and benefit patients around the world.
JOURNAL
Journal of JACC: CARDIOVASCULAR INTERVENTIONS
JOURNAL NUMBER
VOL. 8, NO. 15, 2015
ARTICLE TITLE
Pulmonary Artery Denervation Attenuates Pulmonary Arterial Remodeling in Dogs
With Pulmonary Arterial Hypertension Induced by Dehydrogenized Monocrotaline
ARTICLE PUBLICATION DATE
28 December 2015