We use cookies on your browser to customize content for your viewing and for analytics. If you click “Accept” or continue to browse our website, we assume you have agreed to our use of cookies. For details, please refer to our Cookies Policy.
01 July
153

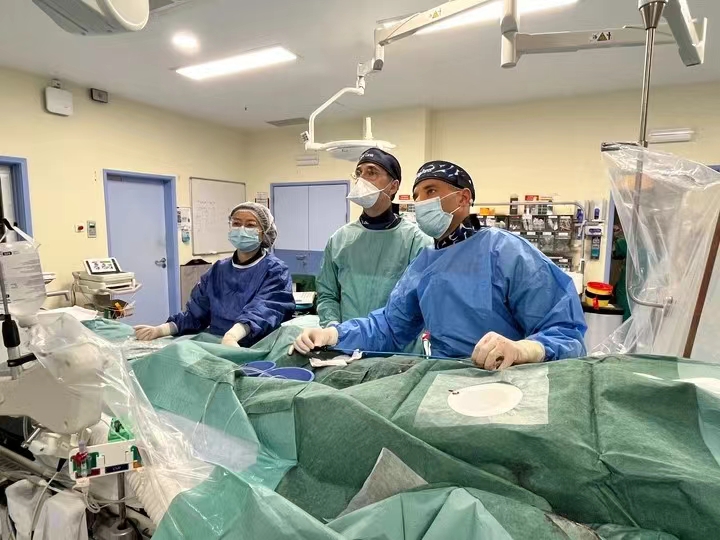
Pulnovo Medical Limited, a globally recognized device pioneer in the treatment of Pulmonary Hypertension (PH) and Heart Failure (HF), is pleased to announce the successful initiation and first two patients enrollment for its global multicenter clinical study exploring the percutaneous pulmonary artery denervation (PADN) treatment for pulmonary hypertension associated with left heart disease. This landmark event took place at Centro Hospitalar Universitário de Lisboa Central - Hospital de Santa Marta in Portugal, marking the beginning of Pulnovo Medical’s innovative product deployment on a global scale.
Under the guidance of Professor Hang Zhang from Nanjing Medical University Affiliated Nanjing Hospital, Prof. Ruben Ramos and his team successfully performed the PADN procedure on two patients. The procedure was smooth, the devices easy to operate, and the results were excellent. The Portuguese medical team was impressed by the PADN technology's safety and efficacy, as demonstrated by many years of outstanding data. Following the successful enrollment, clinical parameters showed promising results when using PADN technology. The products’ comprehensive design and high-precision algorithm control significantly ensured stable energy output and effective surgical results.
The successful first enrollment in this clinical study not only explores medical technology innovation but also aims to further validate the effectiveness and safety of PADN technology, providing better medical services for patients and advancing the development of related disciplines.
 |  |
Prof. Ruben Ramos said:“PADN technology shows great potential in treating pulmonary hypertension associated with left heart disease. We are delighted to be the first in Europe to participate in this global clinical study. Our team is confident in this project and looks forward to bringing more treatment options to European pulmonary hypertension patients through this study. PADN is not only a new treatment method but also brings new hope to patients suffering from pulmonary hypertension. We witnessed significant symptom improvement in patients after the surgery, motivating us to continue advancing this research and strengthening our confidence in PADN’s future applications.”
Jessie Lian,Pulnovo Medical’s CEO said:“The PADN global multicenter clinical study is one of our key projects dedicated to innovative treatment technologies in the cardiovascular field. As an emerging treatment, PADN has enormous potential and prospects. The perfect post-operative results of the first overseas application of PADN reflect the academic borderlessness of the moment. In the future, we will continue to firmly advance our global business, steadily promoting the teaching and learning of PADN technology. We believe this study will further validate the safety and efficacy of PADN technology, bringing hope to more patients. We will also actively expand the global market, strengthening cooperation and exchanges with global partners to promote the development of the global healthcare industry.”
Cynthia Chen, Pulnovo Medical’s Chairlady said:“We are very proud to have successfully completed the first enrollment in the European multicenter clinical study of PADN in Portugal, marking the acceleration of our technology’s global progress. We will continue to promote the global application of PADN, allowing more patients to benefit from this innovative therapy. We believe that the success in Portugal is just the beginning. We will continue to collaborate with renowned cardiovascular experts (KOLs) from multiple European countries, steadily advancing the clinical application of PADN in Europe, Asia, and other countries, further validating and promoting PADN technology. Our goal is to provide better and more treatment options for global patients through continuous innovation and clinical research.”
About PADN
Pulmonary Artery Denervation (PADN) is a percutaneous pulmonary artery intervention procedure that uses a PADN catheter to deliver radiofrequency energy to the sympathetic nerves in the outer membrane of the pulmonary artery, resulting in the disappearance of the myelin sheath of the nerves and the fusion of the axons, which inhibits sympathetic activity, increases cardiac output, reduces pulmonary artery pressure , inhibits the pathologic remodeling of the pulmonary arteries, improves the patient's exercise endurance and cardiac function, and achieves long-term benefits from a single minimally invasive procedure.
About Pulnovo Medical
Pulnovo Medical Limited, is a globally recognized device pioneer in the treatment for pulmonary hypertension and heart failure. Established in 2013 and rooted in innovation, Pulnovo Medical is committed to leveraging our deep expertise in the science of breakthrough technologie with the goal to market our innovative therapeutic solutions and benefit patients around the world.